Scientists pinpoint where thousands of individual proteins are made in intact tissue and single cells
A new technology called RIBOmap can give researchers valuable insight into how protein production in animal and human tissue is altered in disease.
For researchers studying how proteins can cause human disease, knowing precisely where proteins are made within cells and tissues could help them learn about their role in disease and come up with new treatments.
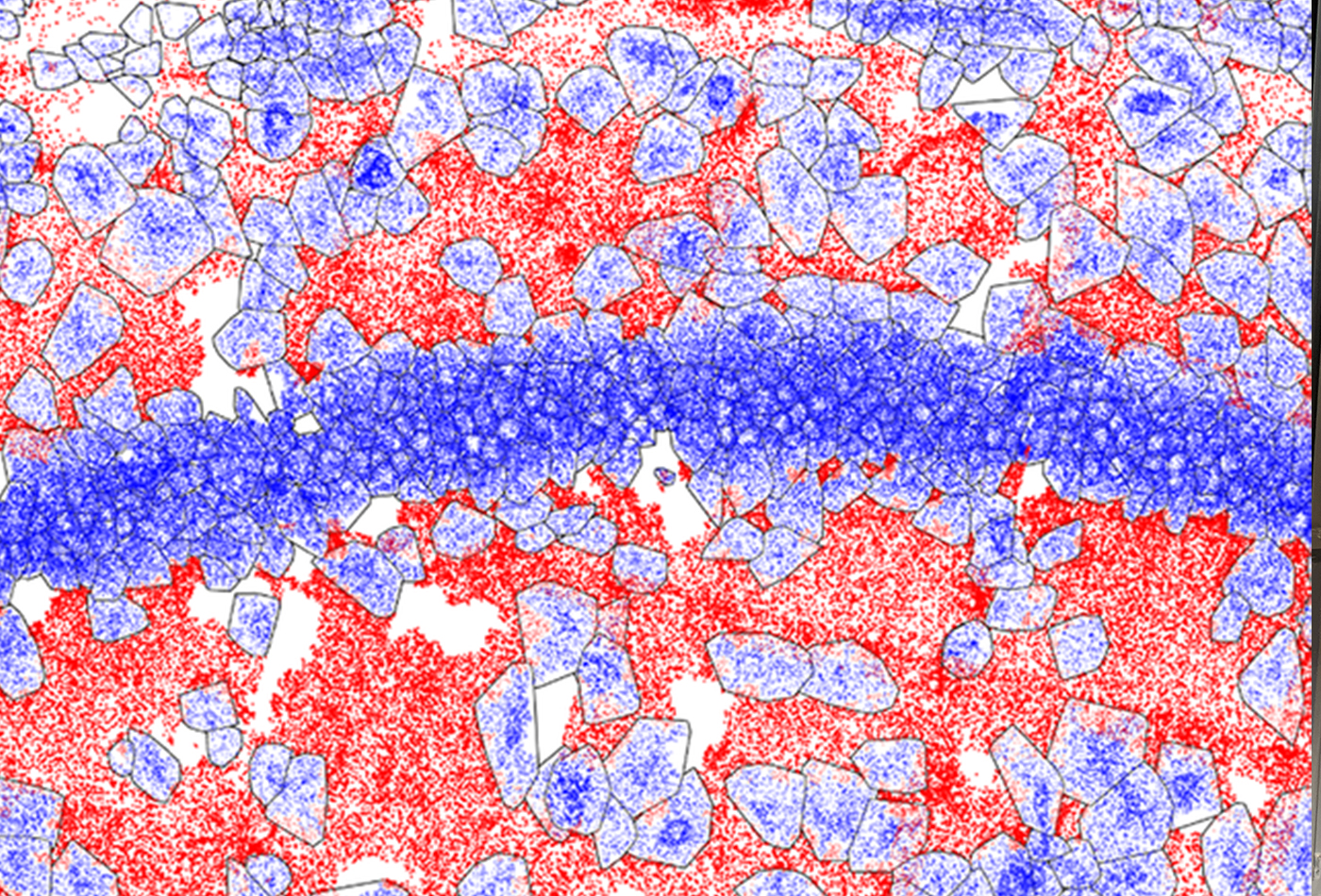
Now, researchers at MIT and the Broad Institute of MIT and Harvard have developed RIBOmap, a technique that lets them pinpoint and visualize the precise locations of thousands of proteins being produced within an intact tissue and even individual cells. Each colored dot in a vast RIBOmap readout represents one mRNA molecule as it is being used to produce the corresponding protein — a process called translation.
The method, described today in Science, provides a way for scientists to learn new details on how translation is regulated within individual cell types and how that changes in disease. In the paper, the team used RIBOmap to study the translation of more than 5,000 genes in mouse brain tissue. They found key differences between where mRNAs are made and where the corresponding proteins are being translated in several cell types, suggesting that cells are regulating translation in ways that existing transcriptomic methods, which analyze mRNA production, aren’t able to detect.
“RIBOmap can reveal spatial patterns in translation at a resolution that has never before been possible,” said Xiao Wang, a core institute member and Merkin Institute Fellow at the Broad and an assistant professor of chemistry at MIT. “When we use RIBOmap to look at individual cells within tissues, we can start to discover how different cell types are regulating translation differently.”
Tool for translatomics
Wang and her lab recognized that scientists needed new tools that focus on translation. Researchers often use levels of mRNA as a proxy for protein levels; in general, an increase in an mRNA molecule within a cell boosts levels of the corresponding protein. However, this is not always a strong correlation: a long-lasting mRNA may be translated into protein over and over, for instance, leading to much higher levels of a protein compared to translation from a short-lived mRNA molecule. Cells also regulate rates of translation — cancer cells, for example, often turn up translation, producing more proteins than healthy cells even when they have similar levels of mRNA.
To develop RIBOmap, the team started with a technique called STARmap that Wang and colleagues previously built to visualize the spatial organization of mRNA molecules within intact tissue. RIBOmap, like STARmap, uses molecular probes that bind to specific mRNA sequences in tissue and single cells. Each probe contains a unique barcode, allowing the researchers to identify each mRNA molecule. And by using a confocal microscope to analyze fluorescent signals generated by in situ sequencing reactions in the tissue sample, the team could map the location of each mRNA. While the STARmap probes can light up any mRNA molecule, the RIBOmap probes only attach to mRNA molecules that are also bound to ribosomes — the cellular machines that carry out translation.
“This means we’re seeing only RNA that is actively being used to produce proteins,” said Jingyi Ren, a co-first author of the new paper and a graduate student in Wang’s lab. “This is incredibly powerful information, because we can start to draw conclusions about which RNA is actually being translated into proteins at any given time and location.”
Running RIBOmap
To test the utility of RIBOmap, the team used it to map where proteins were being made from 5,413 genes expressed in the mouse brain. For some genes, they found STARmap revealed high levels of the corresponding mRNA, but RIBOmap showed that the mRNA was not being actively translated in some regions of the brain. This suggests that cells in those regions are dampening translation of the protein. In neurons, Wang and her colleagues found that RIBOmap generates such high-resolution data that they could even tell where within a single neuron — the central cell body or the long, branched neurites and associated synapses — certain proteins were being made.
“We think this method can be easily applied to tissues other than the brain,” said postdoctoral fellow Hu Zeng, a co-first author of the paper along with Jiahao Huang, a graduate student, both in Wang’s lab. “Because there is no genetic manipulation of cells required, it also means we can easily apply it to isolated human tissues, not just animal models.”
In the future, the researchers imagine using RIBOmap to compare healthy and diseased tissue or see how drugs impact protein production within different cell types or areas of a tissue. They also plan to use the technology to investigate the basic mechanisms cells use to tune levels of translation.