Movassaghi paper published in Organic Letters
The paper, Total Synthesis and Stereochemical Assignment of (−)-Psychotridine was published online on March 17, 2022
A paper authored by Tony Z. Scott, Vinicius F. Armelin, and Professor Mohammad Movassaghi was published in Organic Letters on March 17, 2022.
Total Synthesis and Stereochemical Assignment of (−)-Psychotridine
Tony Z. Scott, Vinicius F. Armelin, and Mohammad Movassaghi
Org. Lett. 2022
Published 17 March 2022
DOI: https://doi.org/10.1021/acs.orglett.2c00448
Abstract
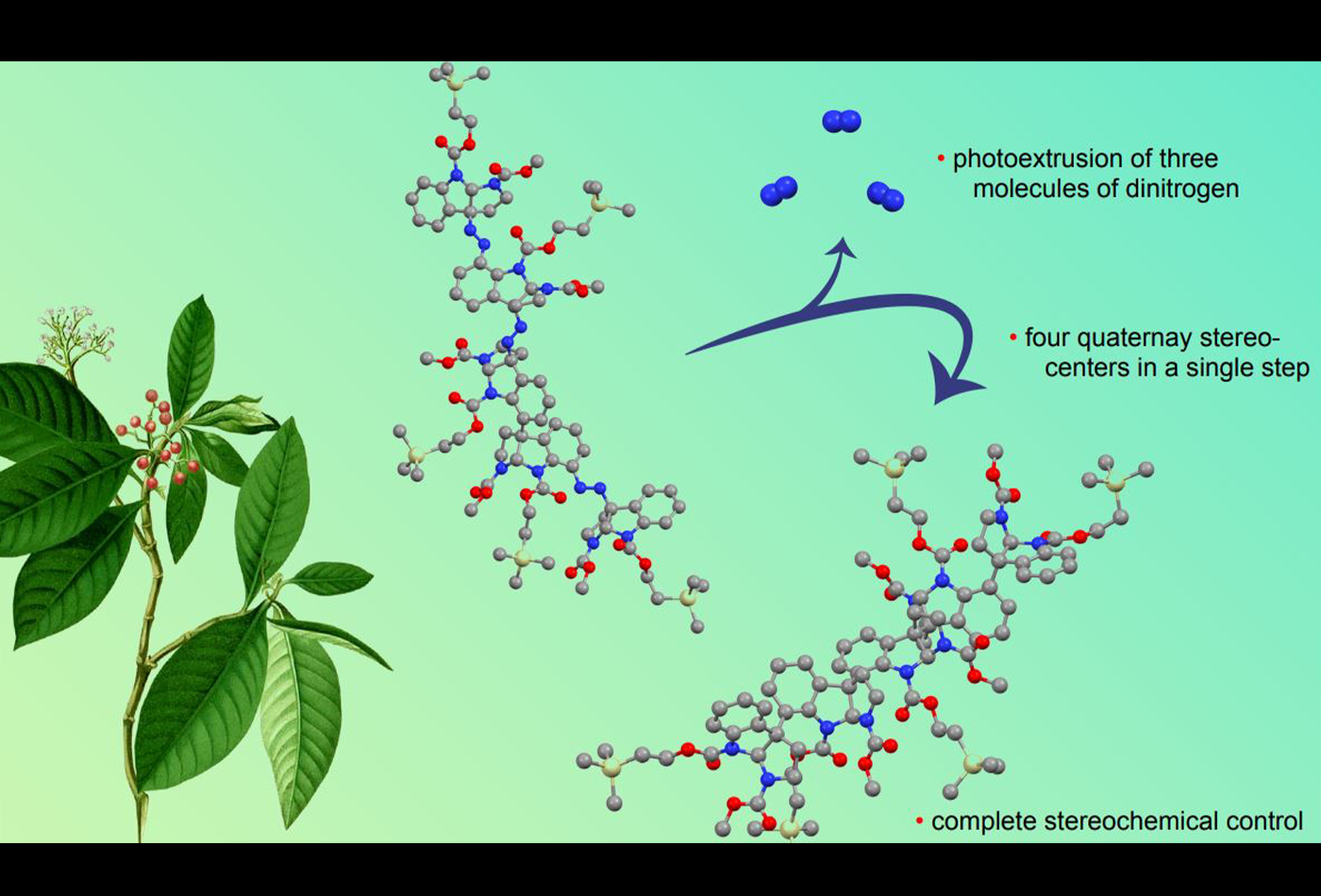
Tony Scott and Vinicius Armelin from the Movassaghi group report the first enantioselective total synthesis and stereochemical assignment of (–)-psychotridine. In a key step, four quaternary stereocenters are secured with complete stereochemical control in a single step via photoextrusion of three molecules of dinitrogen from an advanced trisdiazene pentamer intermediate.
Read the full text on Organic Letters.
The design, discovery, and development of highly selective reactions with broad utility for organic chemistry is central to the research interests of the Movassaghi group. An intimate part of these research activities include mechanistic studies aimed at better understanding the fundamental principles involved in reactivity and selectivity.