Swager paper published in Nature Chemistry
The paper, Dynamic self-correcting nucleophilic aromatic substitution, was authored by Wen Jie Ong and Professor Timothy M. Swager.
A paper authored by Wen Jie Ong and Professor Timothy M. Swager was recently published in Nature Chemistry.
Dynamic self-correcting nucleophilic aromatic substitution
Wen Jie Ong and Professor Timothy M. Swager
DOI: https://doi.org/10.1038/s41557-018-0122-8
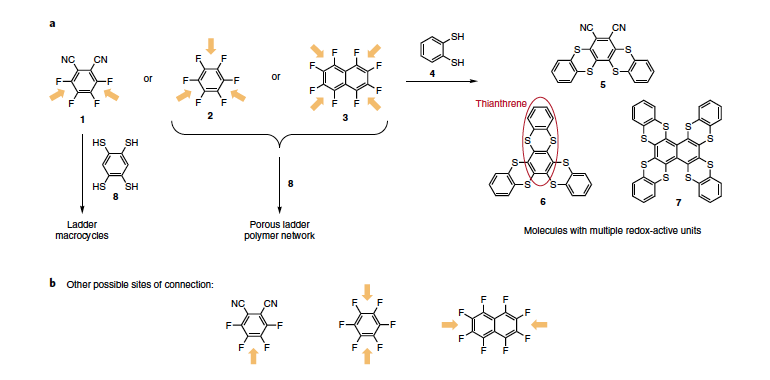
Abstract: Dynamic covalent chemistry, with its ability to correct synthetic dead-ends, allows for the synthesis of elaborate extended network materials in high yields. However, the limited number of reactions amenable to dynamic covalent chemistry necessarily confines the scope and functionality of materials synthesized. Here, we explore the dynamic and self-correcting nature of nucleophilic aromatic substitution (SNAr), using ortho-aryldithiols and ortho-aryldifluorides that condense to produce redoxactive thianthrene units. We demonstrate the facile construction of two-, three- and four-point junctions by reaction between a dithiol nucleophile and three different model electrophiles that produces molecules with two, three and four thianthrene moieties, respectively, in excellent yields. The regioselectivity observed is driven by thermodynamics; other connections form under kinetic control. We also show that the same chemistry can be extended to the synthesis of novel ladder macrocycles and porous polymer networks with Brunauer–Emmett–Teller surface area of up to 813 m2 g−1.
Read the Full Text at Nature Chemistry.
Research in the Swager Group is broadly focused on synthetic, supramolecular, analytical, and materials chemistry. They are interested in a spectrum of topics with an emphasis on the synthesis and construction of functional assemblies. Molecular recognition pervades a great deal of our research. Chemosensors require recognition elements to discriminate chemical signals. Electronic polymers are one of the areas that our group is well known for having made many innovations. The group is constantly developing new electronic structures, properties, and uses for these materials. Recently we have launched an effort to create functionalized carbon nanotubes and graphenes. They have advanced new chemical methods for their functionalization and utilization in electrocatalysis and chemical and radiation sensing. In the area of liquid crystals we make use of molecular complimentarity and receptor-ligand interactions to provide novel organizations.