Surendranath publication receives Concentrate in Chemical & Engineering News
The paper was authored by Megan N. Jackson, Seokjoon Oh, Corey J. Kaminsky, Sterling B. Chu, Guanghui Zhang, Jeffrey T. Miller, and Yogesh Surendranath.
A paper, published in the Journal of the American Chemical Society (JACS) on December 7, 2017 and authored by Professor Yogesh Surendranath and members of his research group, was recognized with a concentrate by Mitch Jacoby in the January 29, 2018 issue of Chemical & Engineering News (C&EN).
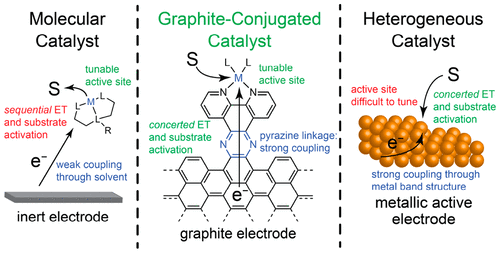
Writes Jacoby: “Most synthetic catalysts fall squarely into one of two categories—homogeneous or heterogeneous, each with its characteristic strong points. Few catalytic systems capitalize on the best of both worlds. Now, a study led by chemists at Massachusetts Institute of Technology takes a key step in that directionHeterogeneous catalysts, especially ones used in electrochemistry, tend to be robust metals that efficiently bind reactant molecules and mediate catalytic reactions via electron transfer. But molecular details of the binding and active sites are tough to pin down and their structures often change during reaction. In contrast, the structures of metallo-organic molecules that serve as homogeneous solution-phase catalysts are well studied and can be modified easily via synthesis to tune catalyst performance. When used as electrocatalysts, however, electron transfer from the electrode through the catalyst to the reagent molecule is a stepwise, thermodynamically unfavorable process. To bypass that problem, a team led by MIT’s Yogesh Surendranath condenses ophenylenediamine derivatives with o-quinone moieties commonly found on graphitic materials. The procedure attaches catalytic molecules to graphite electrodes via strong conjugated pyrazine linkages. The group’s graphite-bound catalysts, which have been used to mediate fuel-cell reactions and convert CO2 to CO, exhibit electron-transfer properties identical to those of heterogeneous metal catalysts but retain the tunability of homogeneous catalysts.”
Strong Electronic Coupling of Molecular Sites to Graphitic Electrodes via Pyrazine Conjugation
Megan N. Jackson, Seokjoon Oh, Corey J. Kaminsky, Sterling B. Chu, Guanghui Zhang, Jeffrey T. Miller, and Yogesh Surendranath
J. Am. Chem. Soc., 2018, 140 (3), pp 1004–1010
DOI: 10.1021/jacs.7b10723
Abstract: Glassy carbon electrodes were functionalized with redox-active moieties by condensation of o-phenylenediamine derivatives with o-quinone sites native to graphitic carbon surfaces. Electrochemical and spectroscopic investigations establish that these graphite-conjugated catalysts (GCCs) exhibit strong electronic coupling to the electrode, leading to electron transfer (ET) behavior that diverges fundamentally from that of solution-phase or surface-tethered analogues. We find that (1) ET is not observed between the electrode and a redox-active GCC moiety regardless of applied potential. (2) ET is observed at GCCs only if the interfacial reaction is ion-coupled. (3) Even when ET is observed, the oxidation state of a transition metal GCC site remains unchanged. From these observations, we construct a mechanistic model for GCC sites in which ET behavior is identical to that of catalytically active metal surfaces rather than to that of molecules in solution. These results suggest that GCCs provide a versatile platform for bridging molecular and heterogeneous electrocatalysis.