Surendranath paper published in ACS Central Science
The paper, Electrochemical Reoxidation Enables Continuous Methane-to-Methanol Catalysis with Aqueous Pt Salts, was published online on June 17, 2019.
A paper authored by Soyoung Kim and Professor Yogesh Surendranath was published in ACS Central Science on June 17, 2019.
Electrochemical Reoxidation Enables Continuous Methane-to-Methanol Catalysis with Aqueous Pt Salts
R. Soyoung Kim and Yogesh Surendranath
ACS Central Science, June 17, 2019
DOI: https://doi.org/10.1021/acscentsci.9b00273
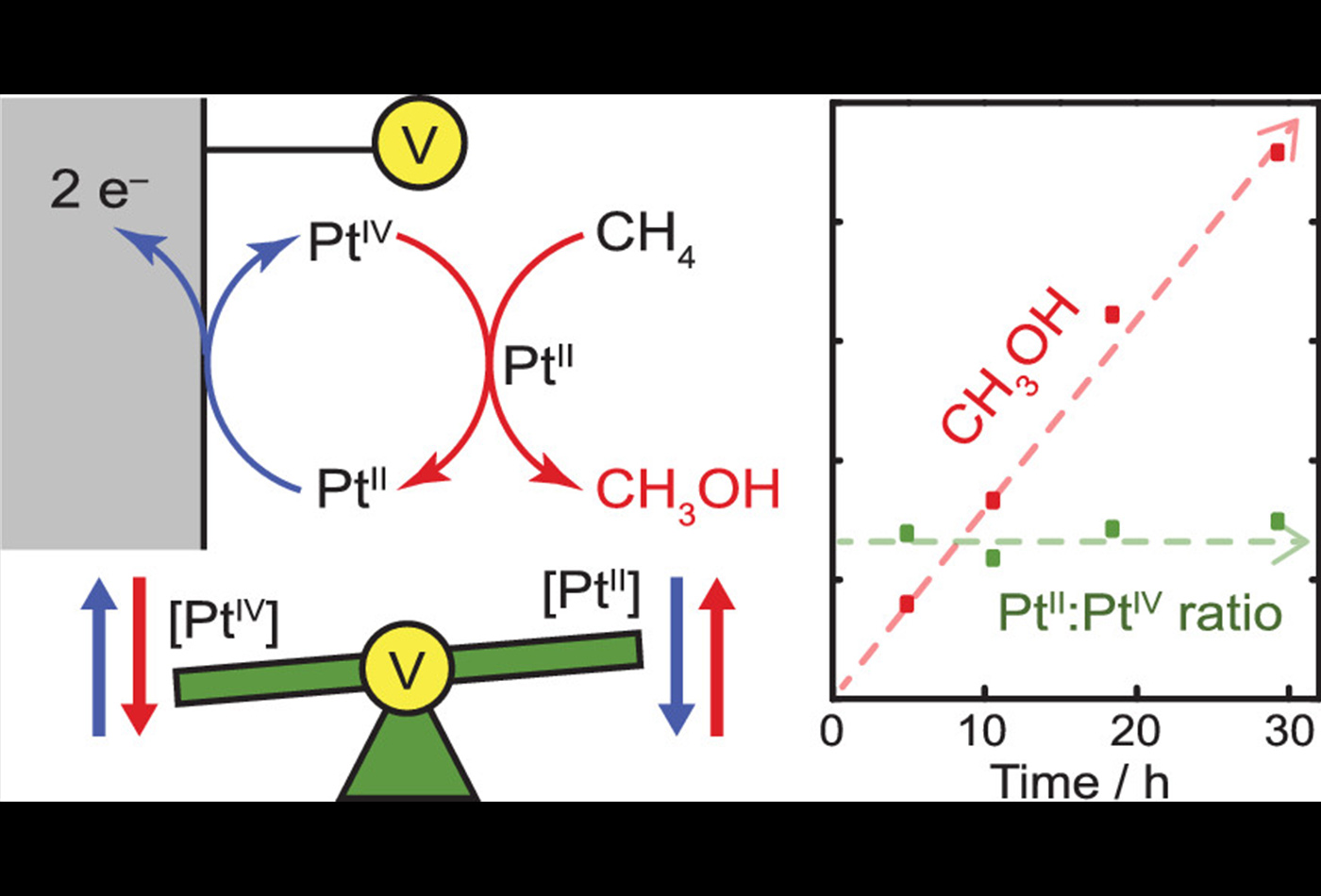
Abstract: The direct conversion of methane to methanol would enable better utilization of abundant natural gas resources. In the presence of stoichiometric PtIV oxidants, PtII ions are capable of catalyzing this reaction in aqueous solutions at modest temperatures. Practical implementation of this chemistry requires a viable strategy for replacing or regenerating the expensive PtIV oxidant. Herein, we establish an electrochemical strategy for continuous regeneration of the PtIV oxidant to furnish overall electrochemical methane oxidation. We show that Cl-adsorbed Pt electrodes catalyze facile oxidation of PtII to PtIV at low overpotential without concomitant methanol oxidation. Exploiting this facile electrochemistry, we maintain the PtII/IV ratio during PtII-catalyzed methane oxidation via in situ monitoring of the solution potential coupled with dynamic modulation of the electric current. This approach leads to sustained methane oxidation catalysis with 70% selectivity for methanol.
Read the Full Text at ACS Central Science.
The Surendranath Lab is focused on addressing global challenges in the areas of chemical catalysis, energy storage and utilization, and environmental stewardship.