Researchers decipher structure of promising battery materials
Family of compounds could someday be useful for fuel cells, supercapacitors, catalysts, and sensors.
A class of materials called metal organic frameworks, or MOFs, has attracted considerable interest over the last several years for a variety of potential energy-related applications — especially since researchers discovered that these typically insulating materials could also be made electrically conductive.
Thanks to MOFs’ extraordinary combination of porosity and conductivity, this finding opened the possibility of new applications in batteries, fuel cells, supercapacitors, electrocatalysts, and specialized chemical sensors. But the process of developing specific MOF materials that possess the desired characteristics has been slow. That’s largely because it’s been hard to figure out their exact molecular structure and how it influences the material’s properties.
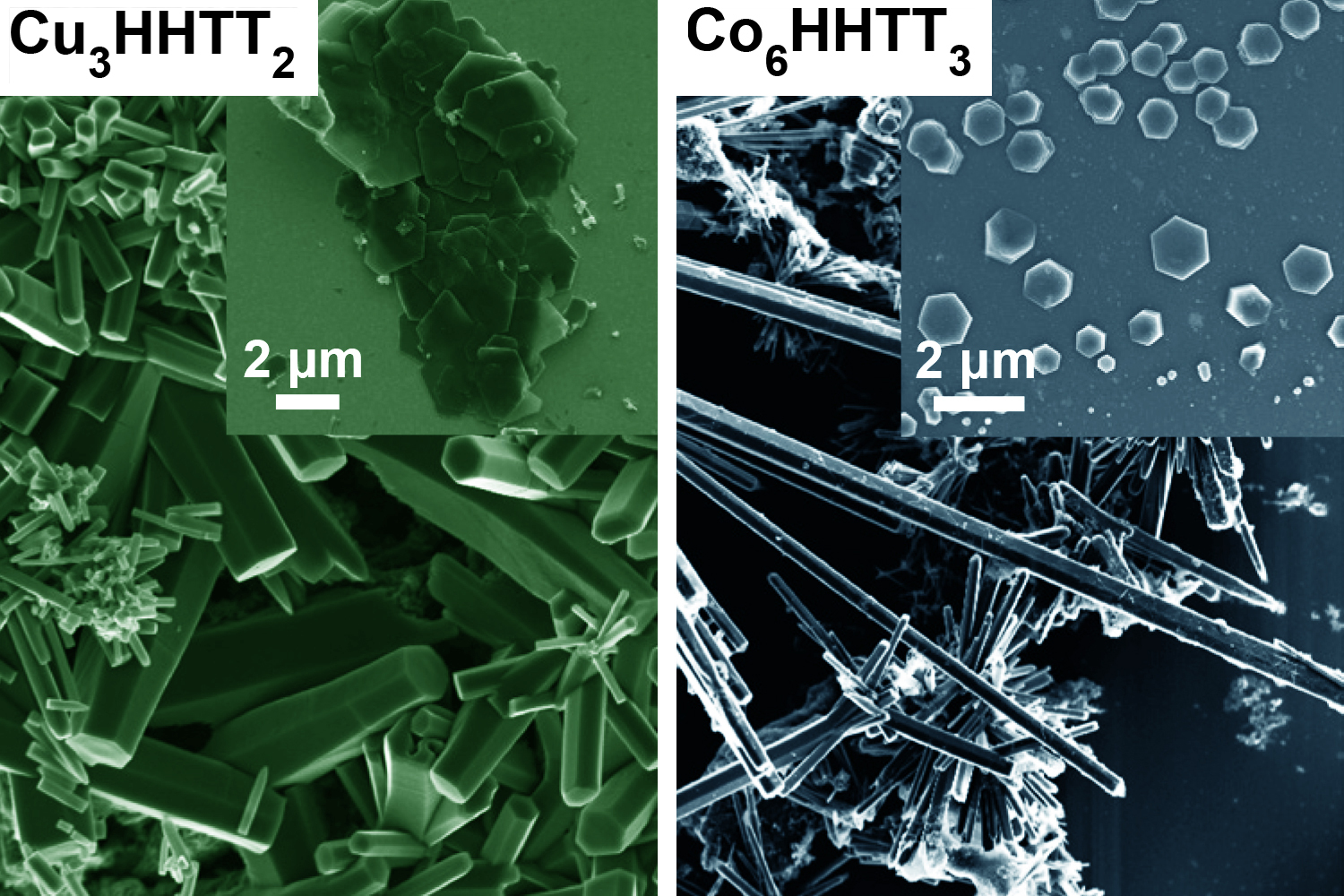
Now, researchers at MIT and other institutions have found a way to control the growth of crystals of several kinds of MOFs. This made it possible to produce crystals large enough to be probed by a battery of tests, enabling the team to finally decode the structure of these materials, which resemble the two-dimensional hexagonal lattices of materials like graphene.
The findings are described today in the journal Nature Materials, in a paper by a team of 20 at MIT and other universities in the U.S., China, and Sweden, led by W. M. Keck Professor of Energy Mircea Dincă from MIT’s Department of Chemistry.
Since conductive MOFs were first discovered a few years ago, Dincă says, many teams have been working to develop versions for many different applications, “but nobody had been able to get a structure of the material with so much detail.” The better the details of those structures are understood, he says, “it helps you design better materials, and much faster. And that’s what we’ve done here: We provided the first detailed crystal structure at atomic resolution.”
The difficulty in growing crystals that were large enough for such studies, he says, lies in the chemical bonds within the MOFs. These materials consist of a lattice of metal atoms and organic molecules that tend to form into crooked needle- or thread-like crystals, because the chemical bonds that connect the atoms in the plane of their hexagonal lattice are harder to form and harder to break. In contrast, the bonds in the vertical direction are much weaker and so keep breaking and reforming at a faster rate, causing the structures to rise faster than they can spread out. The resulting spindly crystals were far too small to be characterized by most available tools.
The team solved that problem by changing the molecular structure of one of the organic compounds in the MOF so that it changed the balance of electron density and the way it interacts with the metal. This reversed the imbalance in the bond strengths and growth rates, thus allowing much larger crystal sheets to form. These larger crystals were then analyzed using a battery of high-resolution diffraction-based imaging techniques.
As was the case with graphene, finding ways to produce larger sheets of the material could be a key to unlocking the potential of this type of MOFs, Dincă says. Initially graphene could only be produced by using sticky tape to peel off single-atom-thick layers from a block of graphite, but over time methods have been developed to directly produce sheets large enough to be useful. The hope is that the techniques developed in this study could help pave the way to similar advances for MOFs, Dincă says.
“This is basically providing a basis and a blueprint for making large crystals of two-dimensional MOFs,” he says.
As with graphene, but unlike most other conductive materials, the conductive MOFs have a strong directionality to their electrical conductivity: They conduct much more freely along the plane of the sheet of material than in the perpendicular direction.
This property, combined with the material’s very high porosity, could make it a strong candidate to be used as an electrode material for batteries, fuel cells, or supercapacitors. And when its organic components have certain groups of atoms attached to them that bond to particular other compounds, they could be used as very sensitive chemical detectors.
Graphene and the handful of other 2D materials known have opened up a wide swath of research in potential applications in electronics and other fields, but those materials have essentially fixed properties. Because MOFs share many of those materials’ characteristics, but form a broad family of possible variations with varying properties, they should allow researchers to design the specific kinds of materials needed for a particular use, Dincă says.
For fuel cells, for example, “you want something that has a lot of active sites” for reactivity on the large surface area provided by the structure with its open latticework, he says. Or for a sensor to monitor levels of a particular gas such as carbon dioxide, “you want something that is specific and doesn’t give false positives.” These kinds of properties can be engineered in through the selection of the organic compounds used to make the MOFs, he says.
The team included researchers from MIT’s departments of Chemistry, Biology, and Electrical Engineering and Computer Science; Peking University and the Shanghai Advanced Research University in China; Stockholm University in Sweden; the University of Oregon; and Purdue University. The work was supported by the U.S. Army Research Office.