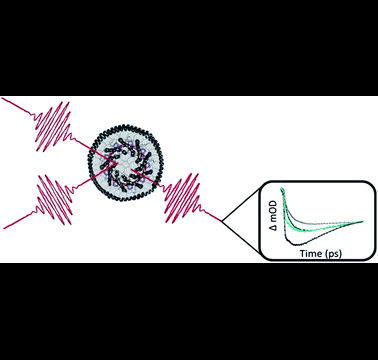
Cao and Schlau-Cohen paper featured on the cover of Chemical Science
Impact of the lipid bilayer on energy transfer kinetics in the photosynthetic protein LH2
A paper authored by Professor Jianshu Cao, Professor Gabriela Schlau-Cohen and members of their respective groups has been highlighted as the cover story of a recent issue of Chemical Science.
Impact of the lipid bilayer on energy transfer kinetics in the photosynthetic protein LH2
John I. Ogren, Ashley L. Tong, Samuel C. Gordon, Aurélia Chenu, Yue Lu, Robert E. Blankenship, Jianshu Cao and Gabriela S. Schlau-Cohen
Chem. Sci., 2018, 9, 3095-3104
DOI: 10.1039/C7SC04814A
Abstract: Photosynthetic purple bacteria convert solar energy to chemical energy with near unity quantum efficiency. The light-harvesting process begins with absorption of solar energy by an antenna protein called Light-Harvesting Complex 2 (LH2). Energy is subsequently transferred within LH2 and then through a network of additional light-harvesting proteins to a central location, termed the reaction center, where charge separation occurs. The energy transfer dynamics of LH2 are highly sensitive to intermolecular distances and relative organizations. As a result, minor structural perturbations can cause significant changes in these dynamics. Previous experiments have primarily been performed in two ways. One uses non-native samples where LH2 is solubilized in detergent, which can alter protein structure. The other uses complex membranes that contain multiple proteins within a large lipid area, which make it difficult to identify and distinguish perturbations caused by protein–protein interactions and lipid–protein interactions. Here, we introduce the use of the biochemical platform of model membrane discs to study the energy transfer dynamics of photosynthetic light-harvesting complexes in a near-native environment. We incorporate a single LH2 from Rhodobacter sphaeroides into membrane discs that provide a spectroscopically amenable sample in an environment more physiological than detergent but less complex than traditional membranes. This provides a simplified system to understand an individual protein and how the lipid–protein interaction affects energy transfer dynamics. We compare the energy transfer rates of detergent-solubilized LH2 with those of LH2 in membrane discs using transient absorption spectroscopy and transient absorption anisotropy. For one key energy transfer step in LH2, we observe a 30% enhancement of the rate for LH2 in membrane discs compared to that in detergent. Based on experimental results and theoretical modeling, we attribute this difference to tilting of the peripheral bacteriochlorophyll in the B800 band. These results highlight the importance of well-defined systems with near-native membrane conditions for physiologically-relevant measurements.