Movassaghi paper published in JACS
The paper, Total Synthesis of (−)-Voacinol and (−)-Voacandimine C, was published online on May 11, 2022
A paper authored by Kristin M. Flynn, In-Soo Myeong, Taylor Pinto, and Professor Mohammad Movassaghi was published in Journal of the American Chemical Society (JACS) on May 11, 2022.
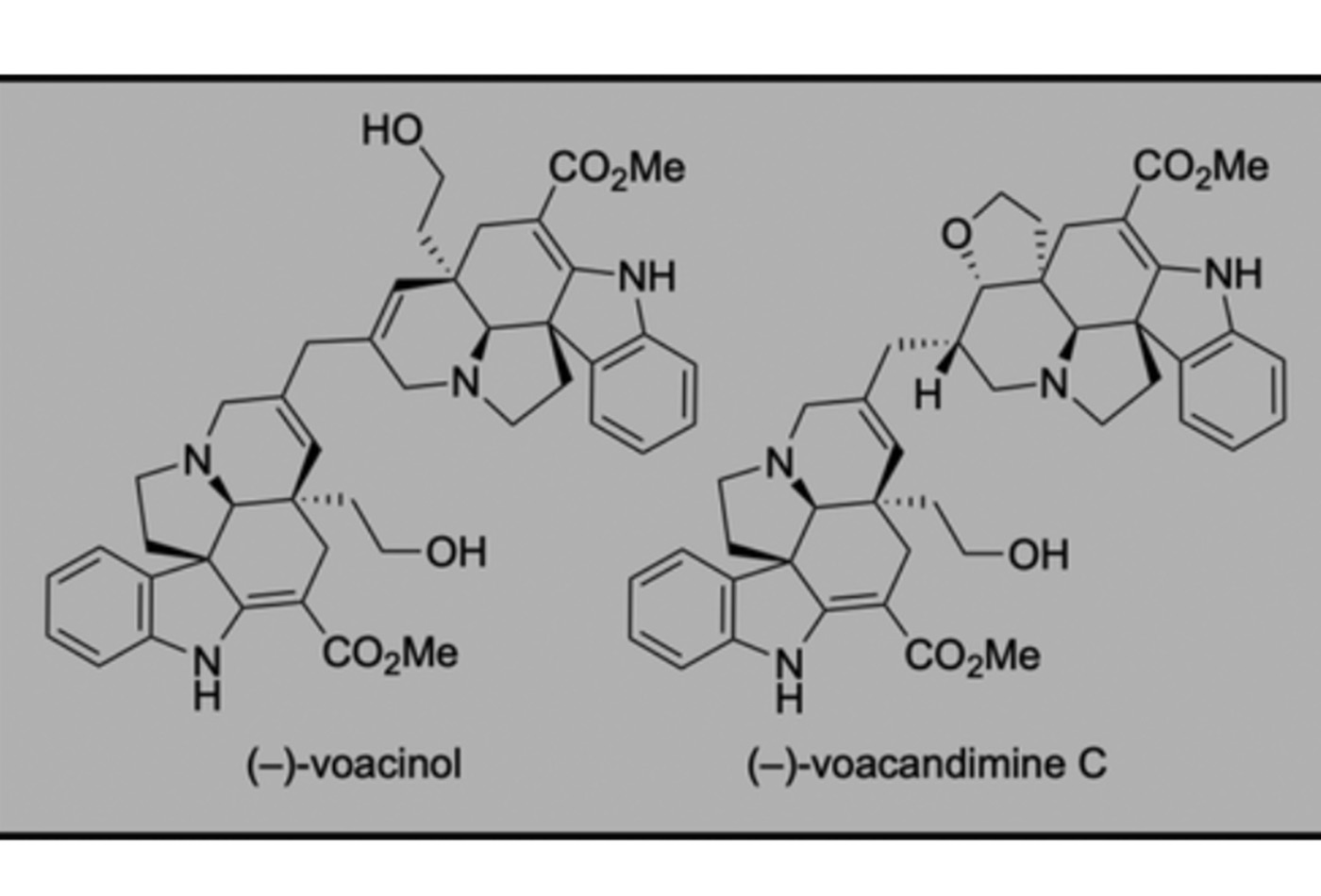
Total Synthesis of (−)-Voacinol and (−)-Voacandimine C
Kristin M. Flynn, In-Soo Myeong, Taylor Pinto
J. Am. Chem. Soc. 2022
Published 11 May 2022
DOI:10.1021/jacs.2c03057.
Abstract
We describe the first total synthesis of complex aspidosperma alkaloids (−)-voacinol and (−)-voacandimine C via a late-stage C7-methylenation strategy inspired by a biogenetic hypothesis. We envisioned rapid access to these natural alkaloids from a common, symmetrical precursor assembled by methylenation of a D-ring-oxidized variant of the structurally related natural product (−)-deoxoapodine. Chemoselective N9-oxidation of a pentacyclic deoxoapodine precursor enabled the synthesis of the corresponding hexacyclic C8-aminonitrile. Stereocontrolled methylenation of a C8-enamine derivative of deoxoapodine, accessed by ionization of the C8-aminonitrile, afforded a symmetrical dodecacyclic bisaminonitrile as a versatile precursor to these bisindole alkaloids. The final-stage, biosynthesis-inspired, controlled reductive opening of the oxolane substructures of this dodecacyclic intermediate provided a unified approach to (−)-voacinol and (−)-voacandimine C, while direct reduction of the same intermediate afforded the structurally related (−)-methylenebisdeoxoapodine.
Read the full text on JACS
The design, discovery, and development of highly selective reactions with broad utility for organic chemistry is central to the research interests of the Movassaghi group. An intimate part of these research activities include mechanistic studies aimed at better understanding the fundamental principles involved in reactivity and selectivity.