How “Tails” Could Make mRNA Therapies Precise, Modular, and More Stable
Researchers from the Wang Group at MIT have developed next-generation messenger RNA (mRNA) therapeutics through chemical synthesis
Researchers from the Wang Group at MIT have developed next-generation messenger RNA (mRNA) therapeutics that demonstrate higher efficiency and stability compared to current technologies. Their method is detailed in the study Chemically Modified mocRNAs for Highly Efficient Protein Expression in Mammalian Cells, published today on ACS Chemical Biology.
Messenger RNA therapies work by providing instructions to a patient’s cells to make proteins that could treat, cure, or prevent diseases. Once the proteins are produced, the cells break down the mRNA, which does not itself alter DNA.
The first mRNA vaccine authorized for use in the United States was for the COVID-19 virus, in which the vaccine provides a “blueprint” that corresponds with a viral protein found on the outer membrane of the virus. The cells create the viral protein on their own, which then activates an immune response—without exposure to the actual virus—and trains the immune system to recognize and attack the virus if it is encountered.
Despite their potential for the healthcare field, mRNAs have relatively short lifetimes, which can limit what their intended uses. For example, while nanogram to microgram ranges of an antigen may suffice to elicit the desired immune response in vaccines; creating antibodies, hormones, or growth factors could require doses of up to gram quantities. And as simply scaling up mRNA quantity could lead to dose-dependent toxicity, researchers have been searching for alternative methods of boosting transgenic protein production without increasing dosage.
Currently known strategies include chemical modification to enhance mRNA vector performance; but certain protein-coding modifications can potentially result in impaired translation, and ribosomal machinery only tolerates a limited range of modifications.
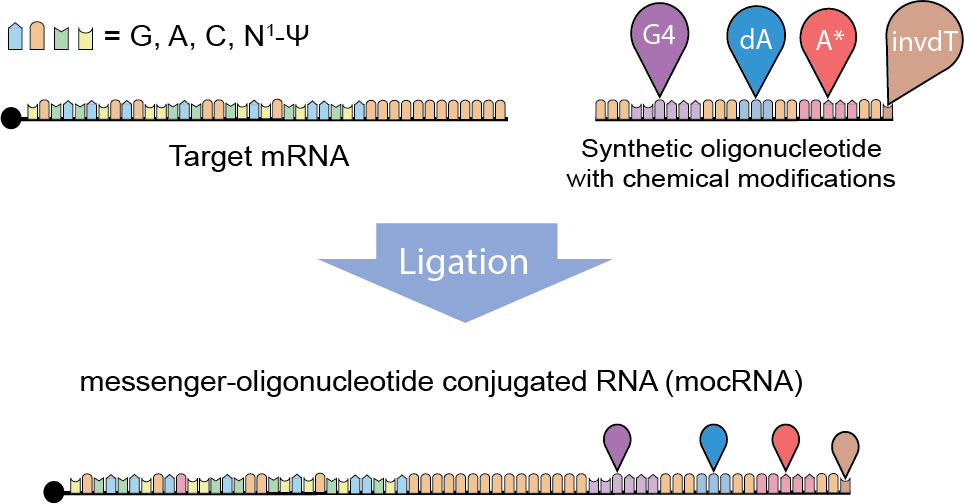
To overcome these limitations, a research group led by Xiao Wang, Thomas D. and Virginia Cabot Assistant Professor of Chemistry at MIT, developed a way to construct messenger-oligonucleotide conjugated RNAs (mocRNAs) with chemically modified tails.
MocRNAs are an mRNA-based expression system with augmented protein production capacity. The Wang Group’s new approach for creating mocRNA is to ligate synthetic oligonucleotides (oligos) with the 3′ ends of mRNAs that contain template-encoded poly(A) tails, a strategy that enables precise and modularized encoding of chemical modifications into RNA vectors.
“These modifications preserve the length distribution of mRNA and demonstrate fewer batch-dependent effects than previous modification strategies. This could potentially be more useful in a therapeutic context,” said Abhishek Aditham, a graduate student in Wang’s lab at MIT and a co-author of the study.
The study demonstrates that in comparison with existing strategies for mRNA modification, mocRNA successfully reduces the RNA degradation rate while increasing therapeutic protein expression in cells.
“The stabilizing effect was observed in cell lines and primary neurons, demonstrating the broad utility of this modular platform,” said Wang, who supervised the study.
Contributing authors are affiliated with the Broad Institute of MIT and Harvard, a research organization comprised of a community of researchers from various disciplines and partner institutions. Read the full paper here.