The research in our group combines elements of organic synthesis, physical organic chemistry and organometallic chemistry to devise catalytic processes of use in solving problems of fundamental importance. In this way we invent or develop new techniques, determine how they proceed and apply them in a synthetically interesting context. The key to our success has been the extraordinary quality of the coworkers in the research group over the years.
We are interested in a wide number of different types of transformations. These include:
The creation and study of new ligands:
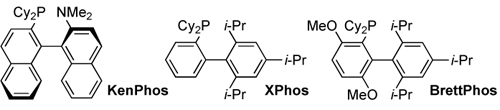
The design of new methods for the formation of carbon-nitrogen bonds:
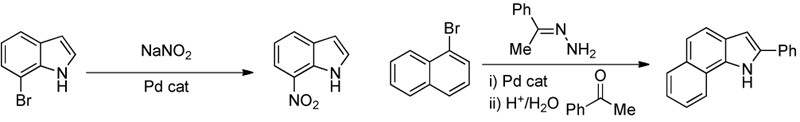
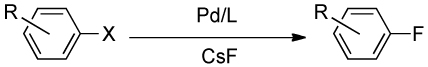
The development of new methods for the formation of carbon-fluorine bonds:
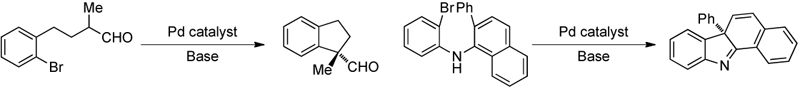
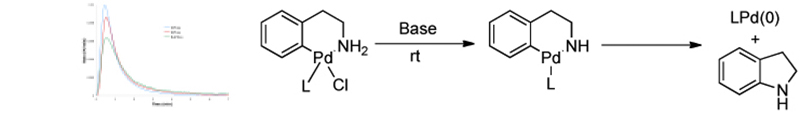
The use of mechanistic and structural studies to aid in ligand and catalyst development:
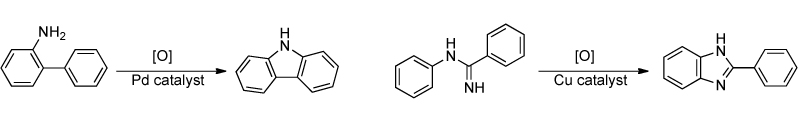
The development of continuous flow chemistry using microreactors and capillary tubing:
The use of mechanistic and structural studies to aid in ligand and catalyst development:
The development of continuous flow chemistry using microreactors and capillary tubing:


